Thermodynamic Database
Components (20)
A total of 20 components are included in the database as listed here:
Al-B-C-Co-Cr-Cu-Fe-Mn-Mo-Nb-Ni-O-Pt-Re-Si-Ta-Ti-V-W-Zr
Suggested Composition Range
The suggested composition range for each element is listed in Table 1. It should be noted that this composition range is based on the validation we performed on commercial alloys. For particular subsystems, the application range may be wider. Some subsystems can be applied to the entire composition range as given in Section Assessed Subsystems.
| Elements | Composition Range (wt.%) |
|---|---|
|
Co |
50-100 |
|
Al, Ni |
0-50 |
|
Cr |
0-30 |
|
Fe, Mo, Re, Ta, W |
0-20 |
|
Nb, Ti, Zr |
0-10 |
|
B,C,Cu,Mn,O,Pt,Si,V |
0-0.5 |
What is new in PanCo2024
Add the new element of O and the O-X binary systems
Phases
Total of 379 phases are included in the current database. The names and thermodynamic models of some phases are given in Table 2. Information on all the other phases is listed in PanCo2024:List of All Phases. Users can also view it through TDB viewer of Pandat™ .
Assessed Subsystems
A total of 277 subsystems, including 187 binary and 90 ternary subsystems have been assessed. The modeling status is indicated by numbers. The systems with number 10 are fully assessed in the whole composition range. The higher value shows higher reliability of the system.
Binary Systems (187)
| Al-B(10) | Al-C(10) | Al-Co(10) | Al-Cr(10) | Al-Cu(10) | Al-Fe(10) | Al-Mn(10) |
| Al-Mo(10) | Al-Nb(10) | Al-Ni(10) | Al-O(10) | Al-Pt(10) | Al-Re(10) | Al-Si(10) |
| Al-Ta(10) | Al-Ti(10) | Al-V(10) | Al-W(10) | Al-Zr(10) | B-C(10) | B-Co(10) |
| B-Cr(10) | B-Cu(10) | B-Fe(10) | B-Mn(10) | B-Mo(10) | B-Nb(10) | B-Ni(10) |
| B-O(10) | B-Pt(10) | B-Re(10) | B-Si(10) | B-Ta(10) | B-Ti(10) | B-V(10) |
| B-W(10) | B-Zr(10) | C-Co(10) | C-Cr(10) | C-Cu(10) | C-Fe(10) | C-Mn(10) |
| C-Mo(10) | C-Nb(10) | C-Ni(10) | C-O(10) | C-Pt(10) | C-Re(10) | C-Si(10) |
| C-Ta(10) | C-Ti(10) | C-V(10) | C-W(10) | C-Zr(10) | Co-Cr(10) | Co-Cu(10) |
| Co-Fe(10) | Co-Mn(10) | Co-Mo(10) | Co-Nb(10) | Co-Ni(10) | Co-O(10) | Co-Pt(10) |
| Co-Re(10) | Co-Si(10) | Co-Ta(10) | Co-Ti(10) | Co-V(10) | Co-W(10) | Co-Zr(10) |
| Cr-Cu(10) | Cr-Fe(10) | Cr-Mn(10) | Cr-Mo(10) | Cr-Nb(10) | Cr-Ni(10) | Cr-O(10) |
| Cr-Pt(10) | Cr-Re(10) | Cr-Si(10) | Cr-Ta(10) | Cr-Ti(10) | Cr-V(10) | Cr-W(10) |
| Cr-Zr(10) | Cu-Fe(10) | Cu-Mn(10) | Cu-Mo(10) | Cu-Nb(10) | Cu-Ni(10) | Cu-O(10) |
| Cu-Pt(10) | Cu-Re(10) | Cu-Si(10) | Cu-Ta(10) | Cu-Ti(10) | Cu-V(10) | Cu-W(10) |
| Cu-Zr(10) | Fe-Mn(10) | Fe-Mo(10) | Fe-Nb(10) | Fe-Ni(10) | Fe-O(10) | Fe-Pt(10) |
| Fe-Re(10) | Fe-Si(10) | Fe-Ta(10) | Fe-Ti(10) | Fe-V(10) | Fe-W(10) | Fe-Zr(10) |
| Mn-Mo(10) | Mn-Nb(10) | Mn-Ni(10) | Mn-O(10) | Mn-Pt(10) | Mn-Re(8) | Mn-Si(10) |
| Mn-Ta(10) | Mn-Ti(10) | Mn-V(10) | Mn-W(6) | Mn-Zr(10) | Mo-Nb(10) | Mo-Ni(10) |
| Mo-O(10) | Mo-Pt(10) | Mo-Re(10) | Mo-Si(10) | Mo-Ta(10) | Mo-Ti(10) | Mo-V(10) |
| Mo-W(10) | Mo-Zr(10) | Nb-Ni(10) | Nb-O(10) | Nb-Pt(10) | Nb-Re(10) | Nb-Si(10) |
| Nb-Ta(10) | Nb-Ti(10) | Nb-V(10) | Nb-W(10) | Nb-Zr(10) | Ni-O(10) | Ni-Pt(10) |
| Ni-Re(10) | Ni-Si(10) | Ni-Ta(10) | Ni-Ti(10) | Ni-V(10) | Ni-W(10) | Ni-Zr(10) |
| O-Pt(5) | O-Re(5) | O-Si(10) | O-Ta(10) | O-Ti(10) | O-V(10) | O-W(10) |
| O-Zr(10) | Pt-Re(10) | Pt-Si(10) | Pt-Ta(10) | Pt-Ti(10) | Pt-W(10) | Pt-Zr(10) |
| Re-Si(10) | Re-Ta(10) | Re-Ti(10) | Re-W(10) | Re-Zr(10) | Si-Ta(10) | Si-Ti(10) |
| Si-V(10) | Si-W(10) | Si-Zr(10) | Ta-Ti(10) | Ta-V(10) | Ta-W(10) | Ta-Zr(10) |
| Ti-V(10) | Ti-W(10) | Ti-Zr(10) | V-Zr(10) | W-Zr(10) |
Ternary Systems (90)
| Al-C-Co(10) | Al-C-Fe(6) | Al-C-Ni(6) | Al-Co-Cr(8) | Al-Co-Mn(8) | Al-Co-Nb(10) |
| Al-Co-Ni(10) | Al-Co-Ta(5) | Al-Co-Ti(5) | Al-Co-W(10) | Al-Cr-Ni(8) | Al-Cr-Ti(8) |
| Al-Fe-Mo(10) | Al-Fe-Ni(8) | Al-Fe-Ta(5) | Al-Mn-Ni(8) | Al-Mn-Si(10) | Al-Mo-Ni(8) |
| Al-Mo-Ti(10) | Al-Nb-Ni(5) | Al-Nb-Ti(10) | Al-Ni-Si(10) | Al-Ni-Ta(7) | Al-Ni-Ti(8) |
| Al-Ni-W(10) | Al-Ta-Ti(10) | C-Co-Cr(8) | C-Co-Fe(8) | C-Co-Mo(6) | C-Co-Nb(6) |
| C-Co-Ta(6) | C-Co-Ti(6) | C-Cr-Fe(8) | C-Cr-Mo(6) | C-Fe-Mn(8) | C-Mo-Ti(8) |
| Co-Cr-Fe(8) | Co-Cr-Mo(8) | Co-Cr-Nb(8) | Co-Cr-Ni(8) | Co-Cr-Ta(2) | Co-Cr-Ti(10) |
| Co-Cr-W(8) | Co-Cu-Fe(8) | Co-Fe-Mn(5) | Co-Fe-Mo(5) | Co-Fe-Ni(5) | Co-Fe-Ta(6) |
| Co-Fe-W(6) | Co-Mn-Ni(8) | Co-Mo-Ni(5) | Co-Mo-Ta(5) | Co-Mo-Ti(5) | Co-Mo-W(8) |
| Co-Nb-Ti(5) | Co-Ni-Si(5) | Co-Ni-Ta(6) | Co-Ni-Ti(5) | Co-Ni-W(10) | Co-Ta-Ti(7) |
| Cr-Fe-Mn(7) | Cr-Fe-Mo(6) | Cr-Fe-Ni(7) | Cr-Mn-Ni(10) | Cr-Mo-Ni(5) | Cr-Mo-Ta(5) |
| Cr-Nb-Ni(10) | Cr-Ni-Si(10) | Cr-Ni-Ti(5) | Cr-Ni-W(10) | Cr-Ta-W(6) | Fe-Mn-Nb(7) |
| Fe-Mn-Ti(6) | Fe-Mo-Ti(5) | Fe-Nb-Ni(8) | Fe-Ni-Si(8) | Fe-Ni-Ti(10) | Mn-Nb-Si(5) |
| Mn-Nb-Ta(10) | Mn-Ni-Si(10) | Mn-Ni-Ta(5) | Mn-Ni-W(5) | Mo-Ni-Ta(6) | Mo-Ni-Ti(6) |
| Mo-Ta-W(6) | Nb-Ni-Ti(7) | Nb-Si-W(5) | Ni-Re-W(5) | Ni-Si-Ti(10) | Re-Ta-W(10) |
Database Validation
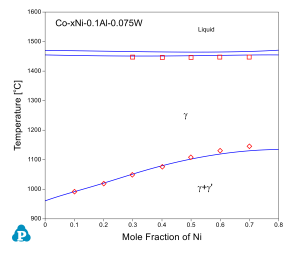
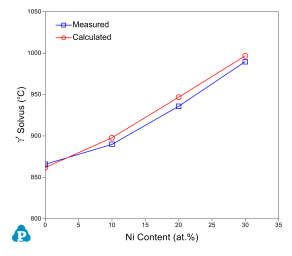
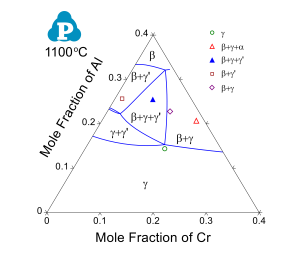
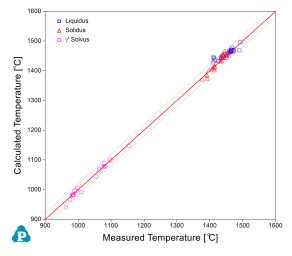
The current thermodynamic database for cobalt alloys has been extensively tested and validated using the published experimental data [2008Ish, 2008Shi, 2010Bau, 2010Pol, 2012Bau, 2015Mak, 2015Mak2]. Figure 1 and Figure 2 show two calculated isopleth in the Co-Al-Ni-W quaternary system compared with the experimental data [2008Ish, 2008Shi]. The isopleth in Figure 1 is a vertical section at 10 at% of Al and 7.5 at% of W and that in Figure 2 is at 10 at.% of Al and 10 at.% of W. In both figures, the calculated phase boundaries are in good agreement with the experimental data. Makineni et al. [2015Mak, 2015Mak2] discovered a new class of γ/γ' Co-based superalloy that is free of tungsten, with the composition of Co-10Al-5Mo-2Nb (in at.%). The solvus temperature can be further enhanced by addition of Ni to form Co-xNi-10Al-5Mo-2Nb, where x can be from 0 to 30 at.%. The comparison between the calculated and measured γ' solvus is shown in Figure 3. Figure 4 and Figure 5 show two calculated isothermal sections with fixed Ni content at 60 at% for the Co-Ni-Al-Cr quaternary system at 1100 °C and 1000 °C, respectively. The calculated results agree well with the experimental observations from annealed alloy samples [2006Bur]. Figure 6 shows the comparison between the calculated liquidus, solidus and γ' solvus temperatures and the experimentally measured ones [2010Bau, 2010Pol, 2012Bau].
Figure 1: Comparison between calculated and measured γ' solvus and solidus temperatures of Co-xNi-10Al-7.5W alloys
Figure 2: Comparison between calculated and measured γ' solvus and solidus temperatures of Co-xNi-10Al-10W alloy
Figure 4: Calculated 1100 °C isothermal section of Co-Ni-Al-Cr system with fixed Ni content at 60 at.% compared with experimental observations
Figure 5: Calculated 1000 °C isothermal section of Co-Ni-Al-Cr system with fixed Ni content at 60 at.% compared with experimental observations
Figure 6: Comparison between calculated results and experimental data for Liquidus, solidus and γ' solvus temperatures
[2006Bur] J. Bursik, P. Broz, J. Popovic, Microstructure and phase equilibria in Ni-Al-Cr-Co alloys, Intermetallics14 (2006): 1257-1261.
[2008Ish] K. Ishida, Intermetallic Compounds in Co-base alloys - Phase Stability and Application to Superalloys, MRS proceedings, 1128 (2008), DOI: http://dx.doi.org/10.1557/PROC-1128-U06-06.
[2008Shi] K. Shinagawa et al., Phase Equilibria and Microstructure on γ' Phase in Co-Ni-Al-W System, Materials Transactions49(6) (2008): 1474-1479.
[2010Bau] A. Bauer, S. Neumeier, F. Pyczak, M. Göken, Scr. Mater. 63 (2010): 1197-1200.
[2010Pol] T.M. Pollock, J. Dibbern, M. Tsunekane, J. Zhu, A. Suzuki, JOM 62 (2010): 58-63.
[2012Bau] A. Bauer, S. Neumeier, F. Pyczak, M. Gken, Creep properties of different gamma'-strengthened Co-base superalloys, Mater. Sci. Eng. A 550 (2012): 333-341.
[2015Mak] S.K. Makineni, B. Nithin and K. Chattopadhyay, Acta Materialia, 85 (2015): 85-94.
[2015Mak2] S.K. Makineni, B. Nithin and K. Chattopadhyay, Scripta Materialia, 98 (2015): 36-39.